Recommendations for Development of Adaptations to Parent-Child Interaction Therapy
PCIT International Board of Directors
Approved: May 1st, 2019
PCIT has broadened in reach and effectiveness through adaptations of many scientist-practitioners with new diagnostic conditions, formats, settings, and target populations. PCIT International welcomes the continued growth of PCIT and seeks to facilitate shared knowledge and collaboration within the PCIT community. Some scientists and practitioners have identified potential gaps in the existing clinical utility and/or research evidence of standard PCIT, and they have made adaptations to the model that they believe may improve outcomes for a specific population or presenting problem. To foster the growth of PCIT, as well as to inform the public about the status of current adaptations, PCIT International’s Board of Directors is proposing recommendations to guide the development, testing, and sharing of information on promising PCIT adaptations. As an organization dedicated to protecting the highest standards of PCIT practice and fidelity, PCIT International can serve a dual role of promoting discourse on the development of evidence-supported adaptations of PCIT and also as a roster or clearinghouse of sorts for information on adaptations in development. Further, a potential role for PCIT International is to endorse evidence-supported adaptations of PCIT.
As an initial step, the PCIT International Board of Directors has generated a list of questions and considerations for developers regarding PCIT adaptations. These recommendations are dynamic – they are a starting point for discussion by the PCIT community, and we invite modifications, clarifications, and enhancements by those interested in adaptations of PCIT.
1. What is the proposed name for the adaptation, and who are the developers and their affiliations? (This identifies the program and persons responsible for it, who could provide further information about the adaptation.)
2. What is the problem addressed by the PCIT adaptation that is not addressed by standard PCIT? (This answers the important question of why the adaptation is needed.)
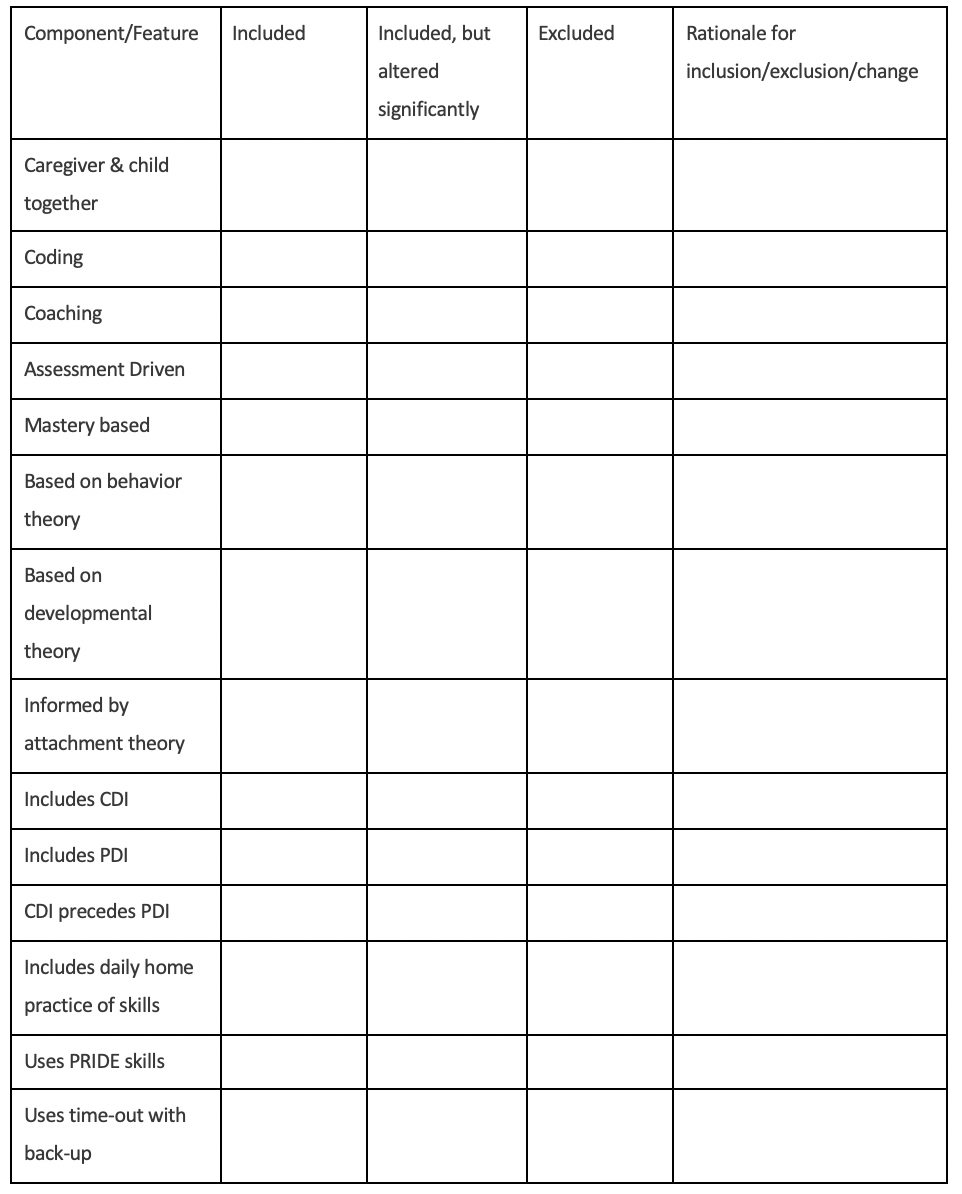
3. How is the adaptation similar to and different from standard PCIT? (This clarifies what specific aspects of PCIT have been adapted or changed, which elements remain the same, the rationale or theory behind the changes made, and how the adaptation is/is not consistent with standard PCIT. The table below provides a suggested list of features to include in this description.)
Approved: May 1st, 2019
PCIT has broadened in reach and effectiveness through adaptations of many scientist-practitioners with new diagnostic conditions, formats, settings, and target populations. PCIT International welcomes the continued growth of PCIT and seeks to facilitate shared knowledge and collaboration within the PCIT community. Some scientists and practitioners have identified potential gaps in the existing clinical utility and/or research evidence of standard PCIT, and they have made adaptations to the model that they believe may improve outcomes for a specific population or presenting problem. To foster the growth of PCIT, as well as to inform the public about the status of current adaptations, PCIT International’s Board of Directors is proposing recommendations to guide the development, testing, and sharing of information on promising PCIT adaptations. As an organization dedicated to protecting the highest standards of PCIT practice and fidelity, PCIT International can serve a dual role of promoting discourse on the development of evidence-supported adaptations of PCIT and also as a roster or clearinghouse of sorts for information on adaptations in development. Further, a potential role for PCIT International is to endorse evidence-supported adaptations of PCIT.
As an initial step, the PCIT International Board of Directors has generated a list of questions and considerations for developers regarding PCIT adaptations. These recommendations are dynamic – they are a starting point for discussion by the PCIT community, and we invite modifications, clarifications, and enhancements by those interested in adaptations of PCIT.
1. What is the proposed name for the adaptation, and who are the developers and their affiliations? (This identifies the program and persons responsible for it, who could provide further information about the adaptation.)
2. What is the problem addressed by the PCIT adaptation that is not addressed by standard PCIT? (This answers the important question of why the adaptation is needed.)
3. How is the adaptation similar to and different from standard PCIT? (This clarifies what specific aspects of PCIT have been adapted or changed, which elements remain the same, the rationale or theory behind the changes made, and how the adaptation is/is not consistent with standard PCIT. The table below provides a suggested list of features to include in this description.)
4. What research evidence has been gathered to demonstrate the effectiveness of the adaptation? (This includes pilot studies, quasi-experimental and/or single-case designs, or randomized-controlled trials of the adaptation. Ideally, it includes a comparison of the adaptation to standard PCIT.)
5. How is the adaptation similar to or different from other adaptations of PCIT addressing a similar problem? (This addresses the uniqueness of the adaptation and its relationship to the broader PCIT-related research literature.)
6. What treatment and/or training protocols and materials have been developed as part of the adaptation? (This provides an indication of the developmental progress of the adaptation and availability of resources for those interested in learning about it in more detail. It also clarifies if these materials are available to be shared with others in the PCIT community.)
7. If PCIT International proceeds to endorse promising PCIT adaptations, here are a few features to be considered:
- The adaptation addresses a problem not addressed adequately by standard PCIT.
c) The adapted program has clear practice guidelines, which may be in the form of a treatment protocol, and guidelines regarding persons qualified to implement it.
d) At least some research evidence for the adaptation is published in peer-reviewed journal articles. Randomized-controlled trials are preferred, but other quasi-experimental and single-case designs will be considered.
e) Applications for endorsement would include a review of the treatment adaptation (theory, methods, training requirements, etc.), development process, research evidence of effectiveness, and fit of the adaptation within the broader treatment outcome literature.
f) Programs with PCIT International endorsement would be featured with their own page on pcit.org, and they would be able to promote their program on pcit.org or link to their own program website from pcit.org.
g) PCIT International endorsement would be renewed periodically to maintain up-to-date information.
The Board of Directors is hopeful that these recommendations will be useful in supporting innovative PCIT’ers who wish to provide effective PCIT-adapted services to more children and families, while protecting PCIT’s core values of being a treatment that is assessment-driven and empirically supported, always. We hope that they spur discussion, suggestions for modifications, and perhaps new connections with like-minded colleagues! We welcome your questions and feedback as we continue to develop useful recommendations for adaptations. If you would like to discuss your ideas with a member of the board, please email [email protected].